Industrial biocatalysis with enzymes is considered a game changer in the development of a sustainable chemical industry. With the help of enzymes, an impressive range of complex molecules such as pharmaceutical agents can be synthesised under environmentally friendly conditions. Researchers at the Karlsruhe Institute of Technology (KIT) have now developed a new class of materials by producing enzymes as foams that have enormous durability and activity. The researchers report on their results in the scientific journal Advanced Materials. A patent application has already been filed for the novel manufacturing process of the enzyme foams.
In order to further develop the field of industrial biocatalysis, which is mainly used in the production of pharmaceuticals and speciality chemicals, researchers are working intensively on new process technologies. In biocatalysis, enzymes instead of chemical catalysts accelerate the reactions, thus saving raw materials and energy. The goal is now to provide enzyme biocatalysts continuously and in large quantities under the gentlest possible conditions. In order to realise efficient material transformations, the enzymes are immobilised in microstructured flow reactors. They are spatially fixed and bound to a reaction-bearing material and thus have limited mobility, which leads to a higher concentration of enzymes and thus to higher productivity.
Foamed Microdroplets from Self-Assembling Enzymes
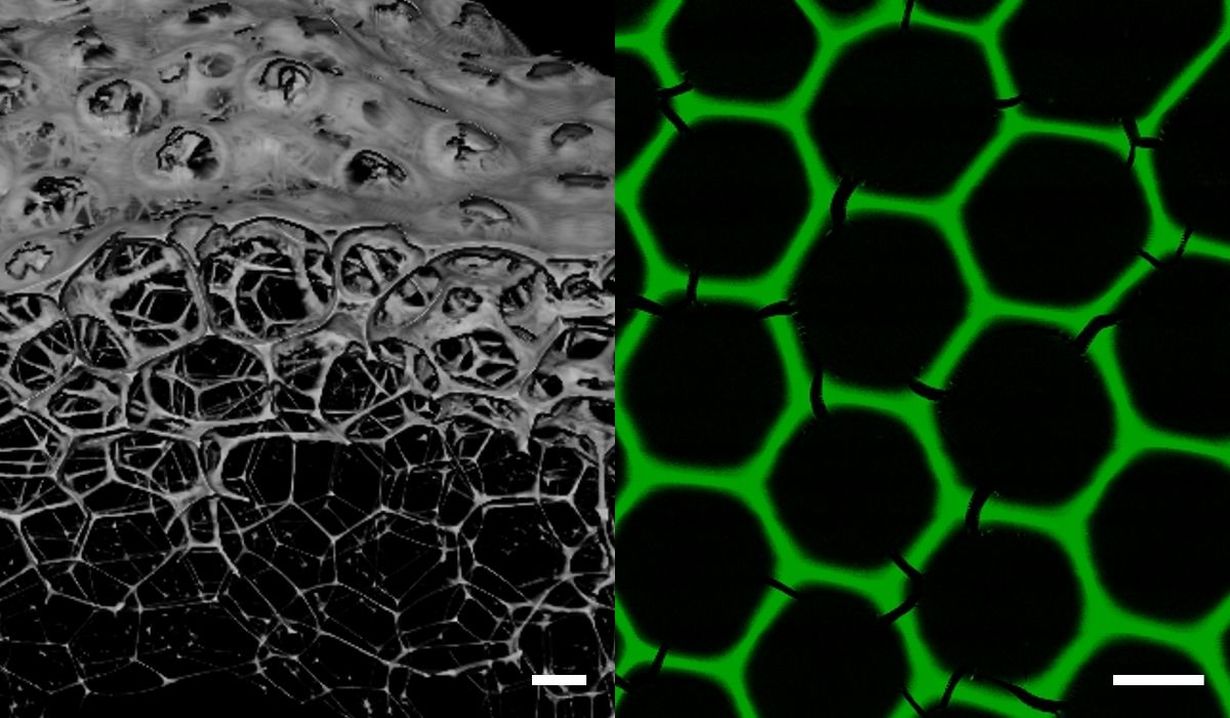
Normally, enzymes change their structure during foaming and thus lose their biocatalytic activity. However, the new protein foams have enormous durability and activity. The activity is a measure of the effectiveness of the enzyme, which ensures that starting materials react with each other as quickly as possible. To make the protein foams, two dehydrogenase enzymes are mixed that carry matching linkage sites so that they can spontaneously form a stable protein network. “A gas flow is then added to this mixture in a microfluidic chip so that microscopic bubbles of uniform size form in a controlled manner,” Professor Christof Niemeyer from the Institute of Biological Interfaces-1 explains the process. The foam of uniform bubble size produced in this way is applied directly to plastic chips and dried, causing the proteins to polymerise and form a stable, hexagonal lattice.
“These are monodisperse full enzyme foams, i.e. three-dimensional, porous networks consisting exclusively of biocatalytically active proteins,” Niemeyer characterises the composition of the new materials. The stable hexagonal honeycomb structure of the foams has a mean pore diameter of 160 µm and a lamella thickness of 8 µm and is formed from the freshly produced spherical bubbles of about the same size after a few minutes.
Efficient Use of Active and Stable Full Enzyme Foams
In order to use enzymes efficiently for substance transformations, they must be immobilised in large quantities under conditions that are as gentle as possible in order to preserve their activity. Until now, enzymes have been immobilised on polymers or carrier particles, but this requires valuable reactor space and can impair activity. “Compared to our already developed full enzyme hydrogels, the new foam-based materials create a much larger surface area where the desired reaction can take place,” Niemeyer describes the major improvement. In contrast to theoretically expected results, the new foams surprisingly show a strikingly high durability, mechanical resistance and catalytic activity of the enzymes, which had not been achieved so far in foaming proteins.
The researchers assume that the stability is due to the matching linkage sites with which the enzymes are equipped. This allows them to join together by themselves and thus form a highly cross-linked lattice during drying, which gives the new material a unique stability. “Surprisingly, the newly developed enzyme foams are significantly more stable after drying for four weeks than the same enzymes without foams,” Niemeyer explains the advantages, “this is of great interest for commercialisation, as it greatly simplifies stock production and shipping.”
The new biomaterials open up versatile avenues for innovations in industrial biotechnology, material sciences or even food technology. For example, the protein foams could be used in biotechnological processes to produce valuable compounds more efficiently and sustainably. The research team was able to show that the foams can be used to produce the industrially valuable sugar tagatose, which is a promising alternative to refined sugar as a sweetener.
Original publication
Julian S. Hertel, Patrick Bitterwolf, Sandra Kröll, Astrid Winterhalter, Annika J. Weber, Maximilian Grösche, Laurenz B. Walkowsky, Stefan Heißler, Matthias Schwotzer, Christof Wöll, Thomas van de Kamp, Marcus Zuber, Tilo Baumbach, Kersten S. Rabe, Christof M. Niemeyer: Biocatalytic Foams from Microdroplet-Formulated Self-Assembling Enzymes. Advanced Materials, 2023. DOI: 10.1002/adma.202303952
Source
KIT, press release, 2023-07-27.
Supplier
Karlsruher Institut für Technologie (KIT)
Share
Renewable Carbon News – Daily Newsletter
Subscribe to our daily email newsletter – the world's leading newsletter on renewable materials and chemicals










