Industrial chemicals and chemical products have been increasingly used for practical societal services such as health care, personal care, food preservation, crop protection, housing, and transportation. In most cases, the use of these chemicals has also implied their immediate or delayed release into the environment, often with unforeseen or unintended consequences. A plea for specialized design phases and production processes of these chemicals, preceding their introduction, to ensure their environmental friendliness.
Two questions
Two questions loom large over our present production processes: why do large-scale releases of chemicals into the environment still occur? And what is the effect of efforts to limit this practice? These questions are particularly pressing in the case of ‘forever chemicals’, such as DDT and PFAS. These form a special category based on their persistence and adverse effects on health and well-being of humans and other organisms.
In recent years, scientific concepts such as exposome and eco-exposome have been developed to systematically characterize, monitor, and measure the adverse effects of chemicals in the environment. At the same time, legislative and regulatory measures have been taken that target all or specific categories of chemicals. Much remains to be done to bring the full life cycle of chemicals in the environment under acceptable control.
Beneficial services
From the 20th century onwards, chemicals have been specifically manufactured to be released into the environment to provide beneficial societal services. Currently, over 350,000 chemicals and chemical mixtures are registered for production and use worldwide, and this number is still growing by many hundreds each year.
The use of chemicals can vary considerably over time, from seconds (the head of a match being struck) to decades (interior paint). Spatial application can also vary from very small areas (toothpaste) to large areas (pesticides). Some chemicals are produced with the goal of release into the environment. Others are environmental pollutants, produced as by-products of industrial production processes and the use of fossil fuels. Like dyes, or sulphur and nitrogen oxides, byproducts of burning fossil fuels.
We can call the entire life cycle of human exposure to the environment (including lifestyle factors) the ‘exposome’. Most pharmaceuticals used in veterinary medicine are released directly into the environment. Examples include the anti-inflammatory drug diclofenac, which decimated vulture populations on the Indian subcontinent; and the antiparasitic drug ivermectin, which interferes with aquatic and terrestrial organisms.
The exposome
Monitoring and measuring the environmental impact of pharmaceuticals such as diclofenac and ivermectin on non-human organisms was one of the reasons for extending the exposome concept to ‘eco-exposome’. But it is clear that innovative R&D aimed at new materials focuses strongly on the user experience and not on the environmental performance of these products. Nevertheless, we can categorize some chemicals as ‘forever chemicals’, because they give rise to the most enduring and difficult to address environmental and health problems. This category includes both chemicals that are introduced into the environment intentionally (e.g. pesticides) and unintentionally (e.g. lead compounds and CFCs).
A time lag between introduction and regulatory measures
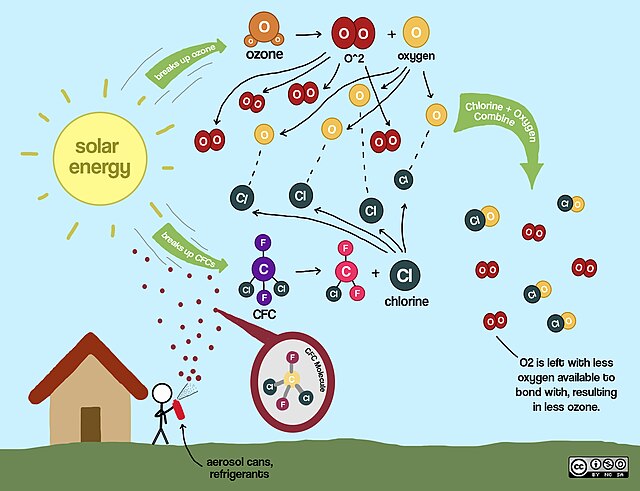
The introduction of chemicals to the market often addresses a pressing societal need. For example, Paul Müller’s discovery of the insecticidal properties of DDT played a major role in the fight against malaria, even leading to Müller’s Nobel Prize in Physiology or Medicine in 1948. The introduction of leaded gasoline in the early 1920s led to improved engine performance and fuel economy in automobiles. The replacement of toxic and flammable refrigerants with CFCs led to the rapid introduction of affordable, safe, and efficient refrigerators in American homes in the 1930s. The benefits of these chemicals were emphasized and their adverse effects were often neglected. For example, Thomas Midgley, who discovered the knock-resistant properties of tetraethyl lead in gasoline in 1921, suffered from lead poisoning as early as 1922.
There was little or no discussion of the potential environmental or social impacts when the chemicals were introduced. Because of these characteristics, there often is a long lag between their commercial and environmental introduction, and the introduction of legal and regulatory measures to address the resulting environmental impacts.
Years of research and sustained lobbying
The phase-out of ‘forever chemicals’ is usually preceded by a long period of observed and reported adverse health and/or environmental effects. Lovelock (1971) reported on CFCs (chlorofluorocarbons) in the atmosphere in 1971, and three years later Molina and Rowland (1974) published their seminal paper on ozone depletion by CFCs. The Montreal Convention, aimed at the phase-out of CFCs, came into force in 1987. By international standards of public policy, this would be considered a rapid process. The opposite is true of lead in gasoline. Lead in gasoline was not phased out until 1970. Leaded gasoline was widely used, and most automotive internal combustion engines depended on it. A possible chemical replacement for tetraethyl lead was the substance MTBE (methyl tert-butyl ether), which was more expensive and also turned out to be a ‘forever chemical’ itself.
Years of research and sustained lobbying were required before the health effects of lead compounds were widely recognized and before societal and regulatory measures directed at the introduction of unleaded gasoline could begin. There were lengthy delays in regulatory and legal action; because of economic interests of global chemical and automotive industries and car owners worldwide. In contrast, effective and less persistent chemical substitutes for CFCs were available at similar cost, and refrigeration manufacturers could adapt their products to the new refrigerants with relative ease.
Persistent Organic Pollutants
In the Stockholm Convention on Persistent Organic Pollutants (POPs) of 2001, it was agreed to phase out DDT and other persistent halogenated compounds such as PCBs. Of all the substances currently covered by the POPs Convention, PFAS compounds are the most difficult to tackle because of their versatility. Per- and polyfluoroalkyl substances (PFAS) are a large group of thousands of synthetic compounds that are used in a very wide range of products, such as personal care products like shampoo and lipstick, waterproof textiles and fire-fighting foam. A common characteristic of ‘everlasting chemicals’: they serve societal functions. Vested commercial interests and a lack of alternatives are just some of the obstacles that stand in the way.
Signing a treaty like the POPs Convention is necessary, but not sufficient. It is just one of many other steps needed for a successful phase-out process. Relevant findings from the EEA reports include the following:
- A. Precautionary measures, often portrayed as a barrier to innovation, could still stimulate innovation, as long as they are supported by adequate regulation and tailored fiscal measures.
- B. More inclusiveness in decision-making is needed to increase public trust. It is notable that the potential role of citizen science was not mentioned.
- C. Better monitoring should reduce the delays between early warnings and action.
- D. The quality and value of risk assessments should be improved.
- E. There should be more general pricing of chemicals based on side effects, following the ‘polluter pays principle’.
Possible solutions
Several proposals have been made for reducing the effect of ever-lasting chemicals. Like designing molecules for degradation as a condition for release and use in the environment. Or ‘chemical simplification’ as a future goal for innovation in chemical science and industry. In a significant recent development, the United Nations Environment Assembly (UNEA) adopted a resolution on the responsible management of chemicals and waste on 2 March 2022. A new IPCC-style Intergovernmental Science-Policy Panel (SPP) on Chemicals, Waste and Pollution Prevention is now being established.
It is noteworthy that there is hardly any mention of enforcement and sanctions. Bans and phase-outs should be taken seriously. Paradoxically, the reason that international treaties such as the POPs Convention could be adopted is precisely because their ratification generally has little effect on the governments involved. By ratifying a treaty, a country voluntarily accepts legal obligations under international law. Without adequate enforcement and effective sanctions, international treaties can remain paper tigers.
Conditions for introduction
Furthermore, it would be best to treat the introduction of all new chemicals in the same way as the introduction of new pharmaceuticals. Binding conditions should be established, together with a monitoring programme under the auspices of the IPPC-like body SPP. This same body should also collect evidence of possible side effects after the chemical has been introduced into the environment, in the same way as the continuous monitoring of pharmaceuticals for possible side effects after their introduction into health care. ‘Chemical simplification’ should be rewarded by preventing the introduction of unnecessary new products.
In conclusion, all of this ultimately points to the need to end the unsustainable production and consumption of chemicals in order to stay within the safe limits of our planet. Although awareness is increasing, education is improving, industrial production is greening, and societal consumption processes and environmental behaviour are improving and adapting, this is not enough. Chemicals can be truly sustainable throughout their life cycle if, and only if, the marketing and use phases are preceded by specialized design phases and production processes that ensure the environmental friendliness of these chemicals.
This is an abbreviated version of the essay: Anton J.M. Schoot Uiterkamp (2025). Environmental fate of chemicals in societal use, Journal of Integrative Environmental Sciences, 22:1, 2507611, DOI:10.1080/1943815X.2025.2507611
Author
Ton Schoot Uiterkamp
Source
Supplier
European Environment Agency (EEA)
Umweltversammlung der Vereinten Nationen (UNEA)
Share
Renewable Carbon News – Daily Newsletter
Subscribe to our daily email newsletter – the world's leading newsletter on renewable materials and chemicals











