Researchers have developed a solar paint that can absorb water vapour and split it to generate hydrogen – the cleanest source of energy.
The paint contains a newly developed compound that acts like silica gel, which is used in sachets to absorb moisture and keep food, medicines and electronics fresh and dry.
But unlike silica gel, the new material, synthetic molybdenum-sulphide, also acts as a semi-conductor and catalyses the splitting of water atoms into hydrogen and oxygen.
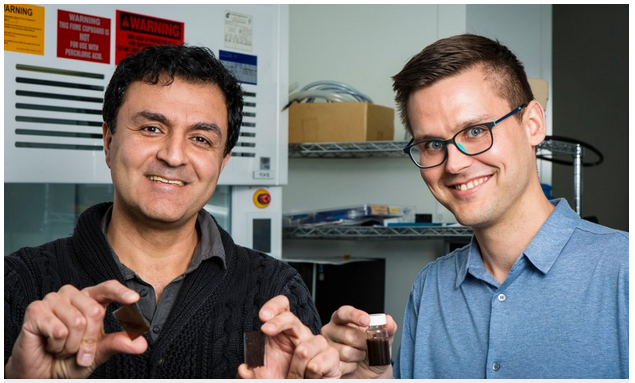
RMIT lead researcher Dr Torben Daeneke said: “We found that mixing the compound with titanium oxide particles leads to a sunlight-absorbing paint that produces hydrogen fuel from solar energy and moist air.
“Titanium oxide is the white pigment that is already commonly used in wall paint, meaning that the simple addition of the new material can convert a brick wall into energy harvesting and fuel production real estate.
“Our new development has a big range of advantages,” he said. “There’s no need for clean or filtered water to feed the system. Any place that has water vapour in the air, even remote areas far from water, can produce fuel.”
His colleague, Distinguished Professor Kourosh Kalantar-zadeh, said hydrogen was the cleanest source of energy and could be used in fuel cells as well as conventional combustion engines as an alternative to fossil fuels.
“This system can also be used in very dry but hot climates near oceans. The sea water is evaporated by the hot sunlight and the vapour can then be absorbed to produce fuel.
“This is an extraordinary concept – making fuel from the sun and water vapour in the air.”
The research has been published as “Surface Water Dependent Properties of Sulfur Rich Molybdenum Sulphides – Electrolyteless Gas Phase Water Splitting” in ACS Nano, a journal of the American Chemical Society. The DOI is 10.1021/acsnano.7b01632.
Source
RMIT University, press release, 2017-06-14.
Supplier
RMIT University (Melbourne, AUS)
Share
Renewable Carbon News – Daily Newsletter
Subscribe to our daily email newsletter – the world's leading newsletter on renewable materials and chemicals