Methanol is of key importance in the sphere of energetical transition from fossil fuels to renewable energy.
The increasing use of methanol as an alternative fuel is quite interesting for the marine industry, due to being liquid at room temperature. This makes methanol transportation and storage a lot less costly than that of gas. Methanol also generates a smaller carbon footprint than any other liquid fuel.
The role of methanol in the industry
Fuel: Methanol can be used as an alternative fuel for internal combustion engines, as a fuel cell, and mixed with gasoline (methanol has a high octane which means that it can improve that of gasoline).
Methanol can be considered a cleaner combustion fuel than fossil fuels such as gasoline and diesel because it produces less air contamination and less greenhouse gas emissions.
Raw chemical material: It is a raw material crucial for producing diverse chemical products such as those we have previously mentioned.
Sustainable methanol production with the use of renewable resources and technologies of carbon capture can reduce the environmental impact of the industry.
Energy storage: It can also be used as a form of energy storage.
It can be produced using renewable energy sources such as air or solar power, through a process called “methanol energy”
This way, excess energy in the form of methanol can be stored. This methanol can then be turned into electricity. Methanol fuel cells can also be stored, and they use methanol as a fuel source to generate electricity through an electrochemical process.
These fuel cells are a promising technology with different applications, such as being a portable energy source, transport, and generating stationary energy.
The four main types of methanol according to the International Council of Clean Transportation (ICCT) are:
- Grey methanol: produced using fossil fuels, mainly natural gas, in a process called steam methane reforming (SMR). During the SMR, the methane (CH4) reacts with steam (H2O) which produces hydrogen (H2) and carbon monoxide (CO) which then turns into methanol.
- Blue methanol: produced using fossil fuels, but with the incorporation of technology that captures and stores the CO2 generated during the production. This means that no CO2 is freed into the atmosphere, which reduces the carbon footprint of methanol production. The term “blue” refers to the captured and stored carbon dioxide.
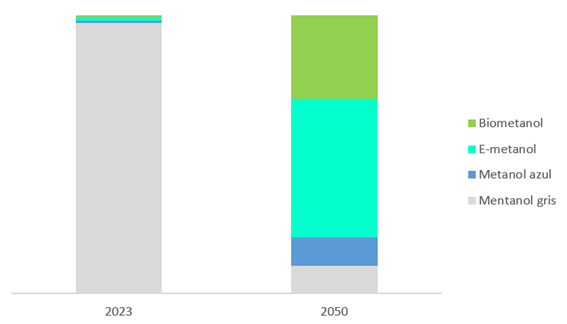
- Green methanol: produced using renewable energy sources such as air, solar or hydroelectric energy. In this process, water electrolysis is used for generating hydrogen which is later used for synthesizing methanol. The production of green methanol does not generate any additional CO2 emissions, which reduces the carbon footprint and helps the environment. There are two types of green methanol: biomethanol, derived from raw biomass materials, and e-methanol derived from renewable electricity from captured CO2.
Green methanol: types and benefits
There are two types of green methanol depending on the origin of their production. The e-Methanol abbreviated to “electromethanol” is an innovative, and environmentally friendly variant of traditional methanol. It is produced using renewable energy without generating contaminating emissions. These are the methods of its production:
- Electrolysis and catalytic hydrogenation: this approach consists of using renewable energy sources such as air and solar energy for generating electricity. Then, that electricity is used for water electrolysis and hydrogen (H2) production. After that, the hydrogen reacts with CO2 in a process of chemical synthesis.
- Hydrothermal processes: The hydrothermal liquefaction of biomass is a process that uses high temperature and pressure to produce liquid bio-oil that can still be processed and turned into e-methanol.
- Solar methanol: The production of solar methanol includes the use of concentrated solar energy for driving chemical reactions that turn CO2 and water into methanol.
Biomethanol, also known as bio-based methanol, or renewable methanol, is produced using raw renewable biomass instead of fossil fuels.
It is considered a sustainable and environmentally friendly alternative to traditional methanol production that uses fossil fuels such as natural gas or carbon. Biomethanol is usually produced using these methods:
- Gasification of biomass: The raw materials of biomass may include agricultural waste, forestall waste, and energy crops in which microwaves were used for fermenting the raw biomass materials for producing methanol. This method usually involves turning sugar or other organic biomass components into methanol, using specifically designed microorganisms.
The benefits of using these two types of green methanol are:
- Reducing the carbon footprint: The production of green methanol from renewable energy sources significantly reduces the carbon footprint associated with methanol production. It mitigates the emissions of greenhouse gases and helps battle climate change.
- Sustainable raw material: As mentioned earlier, e-methanol can be produced using carbon dioxide captured from the atmosphere or industrial processes with water. This means that e-methanol production does not depend on finite fossil fuels, making it a sustainable alternative.
- An energy carrier: green methanol is an efficient carrier of hydrogen that can also be used as a fuel. This makes it valuable for storing and transporting hydrogen, addressing some of the challenges associated with storing and transporting pure hydrogen.
- Energy storage: green methanol can store the excess of renewable energy. During periods of high renewable energy generation, it can be produced using electrolysis and chemical synthesis. Later, when the supply of renewable energy lowers, the stored methanol can once again become electricity or be used for various applications, making the net more stable.
- Versatile raw chemical material: methanol is a raw material of great importance for the chemical industry. This means that chemical products such as formaldehyde, acidic acid, plastics, and synthetic fibers can be made in a more environmentally friendly way and that their production will generate fewer emissions.
- Transport fuels: Green methanol can be used as an alternative fuel for the transport sector. It can be mixed with gasoline and be used for vehicles exclusively fueled by methanol. Methanol vehicles usually produce fewer emissions and contaminants such as nitrogen oxide (NOx) and particles compared to vehicles that use gasoline or diesel.
- Maritime and shipping fuels: green methanol has gained attention as a possible alternative fuel for greener marine vessels, which would reduce the emissions of the marine transport industry.
- Carbon recycling: the production of green methanol and the capture and recycling of carbon dioxide (CO2) from different. This process reduces the concentration of greenhouse gases in the atmosphere.
- Energetic independence: green methanol can be produced using a variety of raw materials, including biomass, natural gas, and carbon dioxide. This versatility can reduce the dependency on a single energetic source and improve energetic security.
- Environmental benefits: the use of green methanol usually generates fewer toxic emissions than other fossil fuels, which improves air quality and reduces health risks, especially in urban regions.
While green methanol plays an important role in the transition to sustainable and renewable energy, it is not without defects.
- Lesser energetic density: methanol has a lesser energetic density compared to gasoline and other conventional liquid fuels. This means that more methanol is needed to produce the same amount of energy, which means that vehicles using methanol may have a lower range.
- More fuel consumption: due to having a lesser energetic density, methanol vehicles will consume more fuel than vehicles that use gasoline. This may raise the costs of using a methanol vehicle.
- Material compatibility: methanol is corrosive and can damage certain components of motors. This may require certain modifications in equipment and materials used in production, which will increase production costs.
Even considering those shortcomings, and most of them have a technological solution, the environmental benefits make green methanol an appealing option in the context of energetical transition.
Green methanol at AIMPLAS
AIMPLAS had various projects centered around obtaining green methanol. One of the most important of these projects is LAURELIN which consists of hydrogenating carbon dioxide to obtain methanol as a fuel for three new technologies of advanced synthesis: magnetic induction, non-thermic plasma induction, and microwave technology. AIMPLAS focuses on the last one of these using a new reactor.
Source
AIMPLAS Blog, press release, 2023-10-25.
Supplier
AIMPLAS (Asociación de Investigación de Materiales Plásticos y Conexas)
Share
Renewable Carbon News – Daily Newsletter
Subscribe to our daily email newsletter – the world's leading newsletter on renewable materials and chemicals









