
As the biofuel industry continues to gain momentum, environmental regulations and demand for biogenic fuels (biofuels) are increasing. Fuels that are produced from modern biomass are called biofuels; most biofuels available in the market today are made from plants (e.g. soy, rape, palm) or waste material (e.g. UCO, tallow, POME) and emit carbon neutral CO2. Certain plants are being cultivated specifically for biofuel production1 including switchgrass, soybeans, and corn in the US, sugarcane in Brazil, cassava and sorghum in China, as well as sugar beet and wheat across Europe.
Many petroleum refineries are co-processing both biofuels and traditional fuels, highlighting the essential nature of quantifying the biogenic proportion of final fuel products. Furthermore, petroleum Naphtha – the intermediary product (by-product) of gasoline, diesel and renewable diesel – is another form of biogenic fuel emerging in the market. As Naphtha is commonly blended with ethanol or gasoline and also used for secondary products (e.g. plastics2,3), calculating the proportion of renewable vs. fossil components is important for compliance and regulatory purposes.
The Radiocarbon Approach
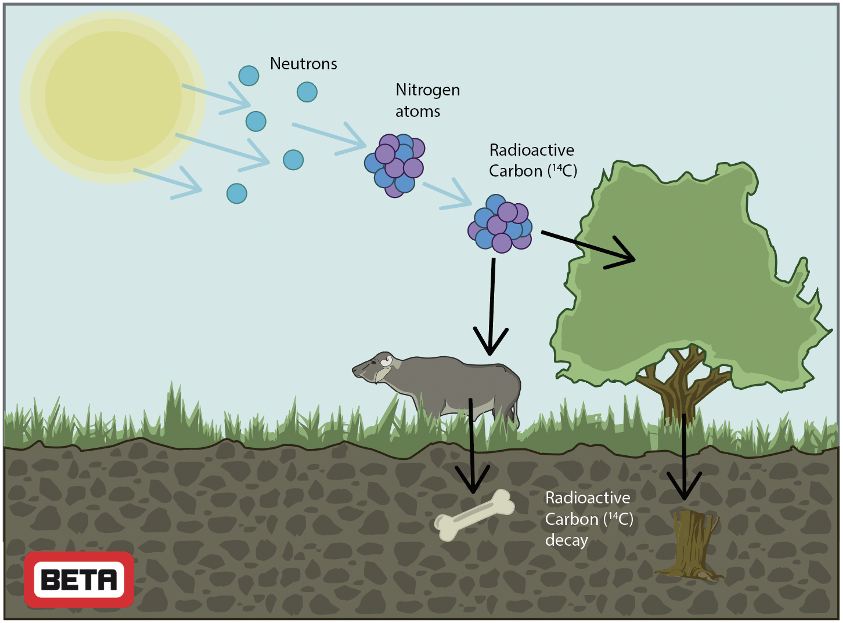
The radiocarbon technique, also known as carbon-14 testing, is based on the fact that various forms of atmospheric carbon are taken up by plants and animals during their lifetime (Figure 1); both the radioactive form (14C) as well as the stable forms (12C, 13C). Thus, a terrestrial plant or animal is in equilibrium with its surroundings, holding a signature of the atmospheric carbon covering the years of its lifetime. Once an organism dies, it ceases to acquire carbon; instead, the radioactive carbon within its biological material will begin to decay. As time passes, the ratio of 14C to 12C gradually decreases, following a known rate. This relationship allows one to estimate time since death using an accelerator mass spectrometer (AMS), comparing the results with the radiocarbon calibration curve.
Thanks to nuclear physics, mass spectrometers have been fine-tuned to separate a rare isotope from an abundant neighboring mass. Today, very small fuel (0.3-0.5mL) and/or feedstock (3-25g) samples are required for measurement, achieving high precision and low background levels – resulting in accurate measurements of biogenic vs. fossil components.
Regardless of the type of fuel or feedstock in question, the radiocarbon method can be utilized – most commonly using the standard ASTM D6866. This standard employs the radiocarbon technique to report sample components in terms of their “pMC” (percent modern carbon). If the material being analyzed is a mixture of present-day carbon and fossil carbon (which contains no radiocarbon), then the pMC value obtained correlates directly to the amount of modern biomass present in the sample. The value reported encompasses a 6% absolute range to conservatively account for variations in end-component radiocarbon signatures, representing maximum values. The ASTM D6866 method determines with excellent accuracy and precision the biogenic carbon fraction of fuels4.
The ASTM D6866 method is employed in a variety of regulations and certification schemes, including:
- United States: EPA’s Renewable Fuels Standard Program (RFS) Program
- United States: California’s Low Carbon Fuel Standard (LCFS)
- Canada: O. Reg. 226/18 (Greener Diesel)
- FRANCE: Bio-content determination by 14C for HVO import specified by Customs (Circulaire TIRIB)
- NETHERLANDS: 14C required for determination of HVO bio-content
Conclusion
There are a variety of different points within the process of developing biofuels in which the radiocarbon method can be readily applied, including:
- Validating the content of biofuel components
- Investigating the yield of production (e.g. naphtha, kerosene, diesel)
- Demonstrating biogenic content within a refinery fuel pool
- Verifying the proportion of biogenic material in proportion to the total fuel content
This standard method can be applied to other products and initiatives, including quality control for natural (vs. synthetic alternatives) and biobased products.
References
- Tudge, S.J., Purvis, A. and De Palma, A., 2021. The impacts of biofuel crops on local biodiversity: a global synthesis. Biodiversity and Conservation, 30(11), pp.2863-2883.
- Sarker, M., Rashid, M.M. and Molla, M., 2011. Waste plastic conversion into chemical products like naphtha. Journal of fundamentals of renewable energy and applications, 1. DOI: 10.4303/jfrea/R110101
- Shaw, D.K. and Sahni, P., 2014. Plastic to oil. Journal of Mechanical and Civil Engineering, pp.46-48.
- Haverly, M.R., Fenwick, S.R., Patterson, F.P. and Slade, D.A., 2019. Biobased carbon content quantification through AMS radiocarbon analysis of liquid fuels. Fuel, 237, pp.1108-1111.
Author
Maren Pauly (Beta Analytic)
Source
Beta Analytic, press release, 2o22-10.
Supplier
Share
Renewable Carbon News – Daily Newsletter
Subscribe to our daily email newsletter – the world's leading newsletter on renewable materials and chemicals









